A Biocompatible Lossen Rearrangement in Escherichia Coli
Forget your fume hood, E. coli just became your new reaction vessel
💡 What’s new?
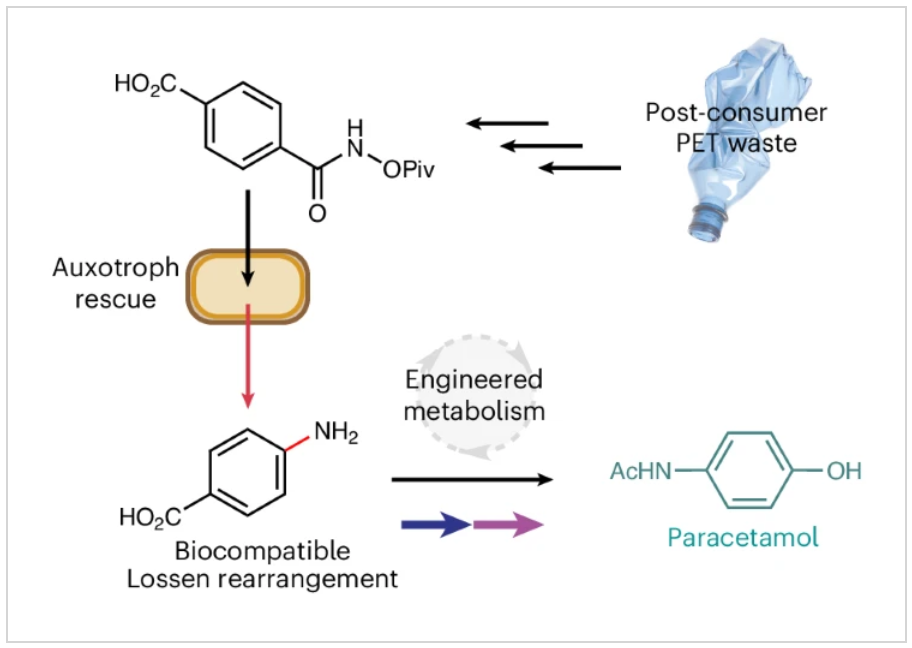
Researchers have shown that a classic organic transformation (the Lossen rearrangement) runs smoothly inside E. coli cells. The reaction uses only phosphate as a catalyst, so no engineered enzyme is needed.
By converting a specially activated precursor into para-aminobenzoic acid (PABA), the reaction restores growth to an E. coli strain that cannot make PABA on its own. This confirms the chemistry really happens inside live cells (verified by the standard cell-growth measurement, OD₆₀₀).
The same precursor can be made from recycled PET plastic, letting the microbe turn waste into valuable products such as paracetamol when the reaction is linked to other metabolic steps already present in the cell or introduced from other organisms.
💡 Why this matters
Expands the metabolic toolbox – You can now plug a purely chemical step into microbial pathways without engineering a catalyst, opening new routes to specialty chemicals and drug intermediates.
Reduces process steps – Moving a rearrangement into the fermentation broth avoids separate reactor stages and cuts solvent use.
Enables circular chemistry: Demonstrates direct conversion of PET plastic waste into pharmaceutical starting materials inside living cells.
➡️ Full article: https://lnkd.in/ejcgTHVh
➡️ Leave your comment on my LinkedIn here
Johnson, N.W., Valenzuela-Ortega, M., Thorpe, T.W. et al. A biocompatible Lossen rearrangement in Escherichia coli. Nat. Chem. 17, 1020–1026 (2025).
#SyntheticBiology #MetabolicEngineering #GreenChemistry #Bioorthogonal #Biotech #Pharma #DrugDiscovery #SmallMolecules