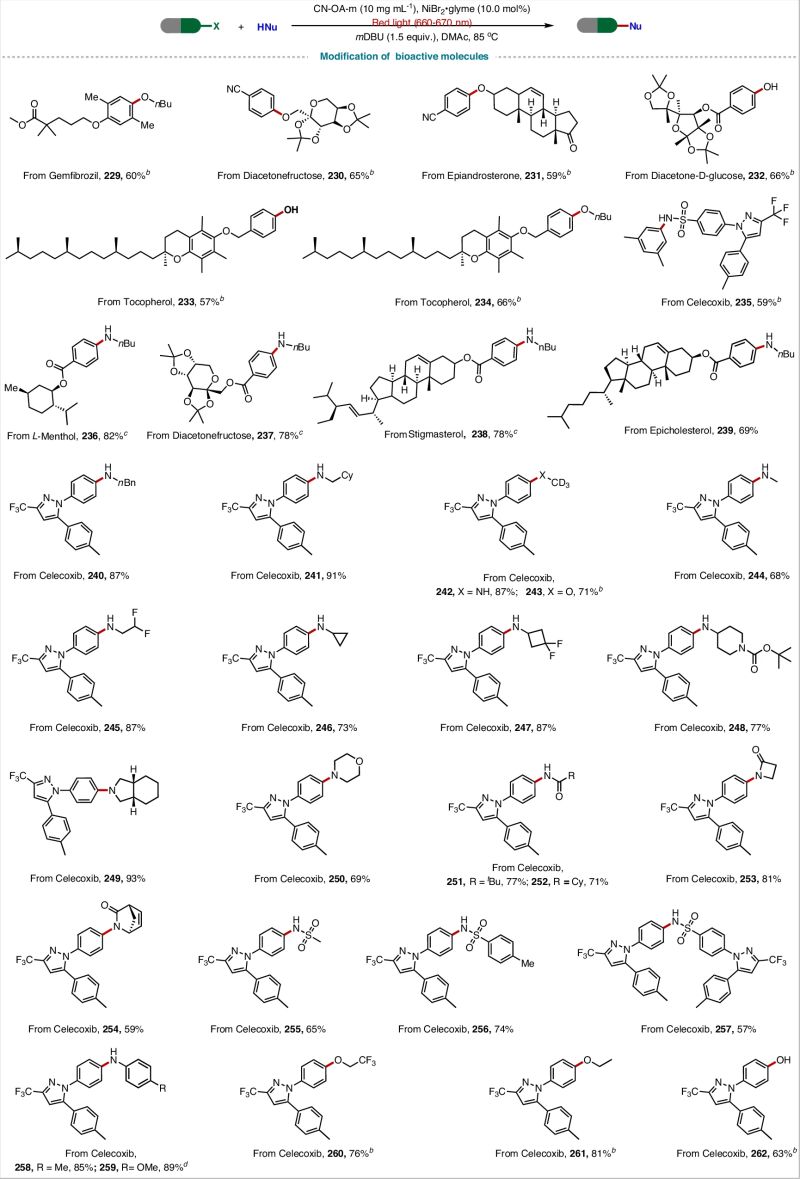
Carbon–Heteroatom Cross-Coupling via Red-Light Metallaphotocatalysis
Recently published paper in Nature Communications claims (as the title says):
“General method for carbon–heteroatom cross-coupling reactions via semiheterogeneous red-light metallaphotocatalysis”
Quite a bold statement, so let’ take a look at it.
Key highlights include:
✅ Red light penetrates 23x deeper than blue light – enabling gram-scale reactions in 10 cm flasks
✅ Four bond types (C-N, C-O, C-S, C-Se) under identical conditions – no more case-by-case optimization hell
✅ 200+ substrates (!) with yields up to 94% – including challenging bioactive molecules like celecoxib and gemfibrozil
✅ Recyclable catalyst (CN-OA-m) that works for 5+ cycles
✅ They even synthesized tetracaine in 85% yield at gram scale under solar light
How they achieved that:
They’re using polymeric carbon nitride as a semiheterogeneous photocatalyst with nickel, operating via a Ni(I)/Ni(III) cycle. The red light (660-670 nm) prevents the substrate photodegradation that plagues blue-light systems.
🔬 Is it really breakthrough in Red-Light Photocatalysis and cross coupling reactions?
I think the substrate scope is impressive. But are you going to use it if you can find a (potentially better) procedure which is more tailored for your specific transformation?
How applicable is this protocol for high throughout experimentation?
Are you going to use it for your synthetic route? I would be curious to hear how it works for you.
➡️ Full article: https://www.nature.com/articles/s41467-025-61812-z
➡️ Leave your comment on my LinkedIn here

#Catalysis #PhotoRedox #ProcessChemistry #MedicinalChemistry #OrganicSynthesis #Drugdiscovery